Biopharmaceutical
Whether you need nutrients for your formulated media, cGMP chemicals for your downstream purification or multicompendial excipients for your final formulation, Spectrum understands that every process is different. That is why we offer custom, batch-specific packaging, additional testing for specific trace impurities or supply from a certain original manufacturer to support your unique needs. We are able to take a consultative approach with you concerning your projects, small and large. Spectrum has been providing value added chemical solutions to our customers in the pharmaceutical industry for the past 50 years, and we continue to invest in every facet of our operations to provide current and new customers’ solutions for the next 50 years.
In House Laboratory Testing
Our extensive laboratory testing meets the highest standards with the most modern instrumentation in the industry. Spectrum’s analytical laboratories are fully equipped with state-of-the-art technology supported by best practices, current Good Laboratory Practice (cGLP) and current Good Manufacturing Practice (cGMP). All of Spectrum’s regulated pharmaceutical grade chemicals are tested, processed and warehoused under current cGMP per 21CFR parts 210 and 211 in FDA registered facilities in both New Jersey and California. Learn More
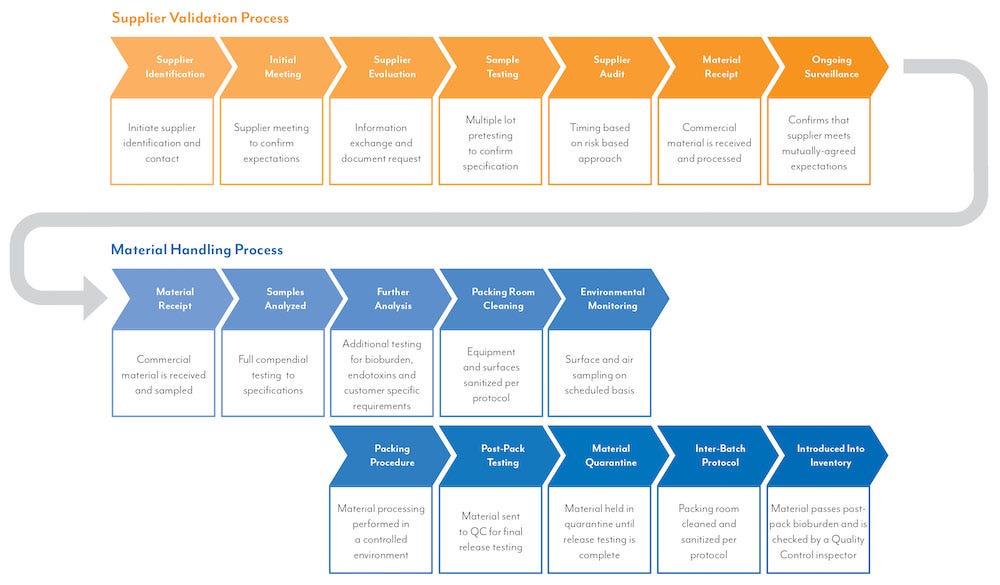
Our Processes

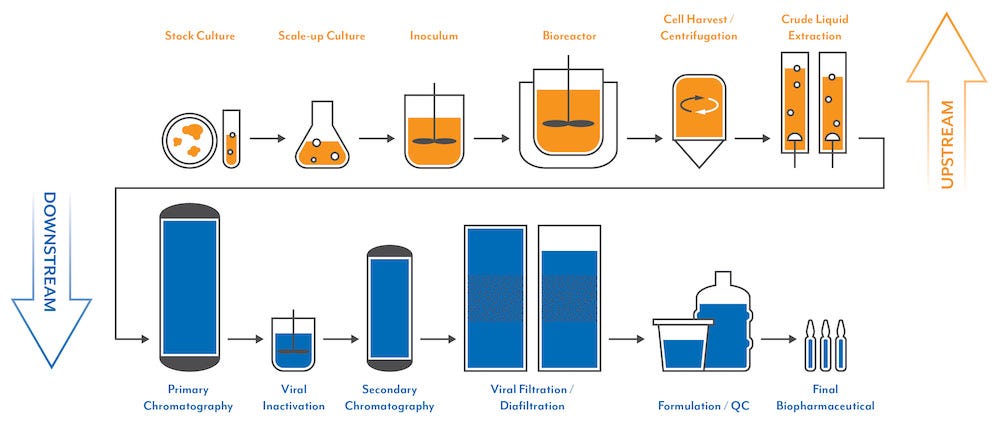
Upstream / Downstream Processing
Different phases of your process have different needs, such as chemical type, regulatory oversight and product quantity. Whether you need several hundred grams as an additive to your custom media formulation, metric tons for your downstream purification buffers and salt gradients or several hundred kilograms of a multicompendial excipient to be used in your final parenteral formulation, our goal is to provide the appropriate product for the specific intended use. We understand that no two customers or processes are identical, and we will work with you to support your unique needs.

The Right Materials from the Start
Being a value-added supplier to the biopharmaceutical and parenteral pharmaceutical markets means starting with the right manufacturing partners. Spectrum supply chain professionals in North America, China and India work with the top manufacturers around the globe, who are committed to working with us. This allows both large and small customers to leverage our sourcing capabilities and contacts in order to receive the best product for specific current and future needs.
Our Products
Spectrum supplies over 100 unique cell culture additives throughout the world and encourages you to review our current offering for your upstream media needs. Our product offering covers everything from research grade chemicals for upstream R&D and cell culture, through cGMP chemicals for scale-up and production.
If you do not see the product, grade or form of the ingredient you require, just contact our chemical specialist at 1 (800) 815-8742.





