BioHale® high purity, low endotoxin excipients.
Manufactured by DFE Pharma, our BioHale® portfolio includes BioHale® Sucrose and BioHale® Trehalose Dihydrate, which are ideal for parenteral, inhalation, oral and ophthalmic applications.
Unlike taking medications orally or via inhalation, introducing a drug into the body by parenteral administration poses greater risk, since the body’s natural defenses are bypassed. With guaranteed high purity and low endotoxin levels, BioHale excipients enables the stabilization of biological molecules in biopharmaceutical formulations. BioHale is widely used in several current vaccines as part of the fight against COVID-19.
Uncompromised Quality
- High purity and low endotoxin excipients facilitate the stabilization of biomolecules to help reduce the loss of activity and improve efficacy
- Multi-compendial specifications compliant with USP-NF, JP, ChP monographs
- Produced in state-of-the-art, FDA-inspected manufacturing facilities in accordance with ICH Q7, which provides cGMP guidance for the manufacturing of active pharmaceutical ingredients (APIs)


Security of Supply
-
Smooth supply of BioHale excipients with proven track record of delivery
-
Active purification steps ensure excipients are independent of raw material variation
-
Production capacity with full control of process

BioHale Sucrose (Beet Derived)
BioHale Sucrose is a non-reducing crystalline disaccharide made up of glucose and fructose. It is well suited to provide solution-state stabilization, as well as cryo and lyo-protection for biomolecules to be used in various administration forms, such as for parenteral or ophthalmic.
Stabilizing Agent
BioHale Sucrose is a non-reducing sugar and does not react with amino acids or proteins, inhibiting the Maillard reaction. BioHale Sucrose provides solution-state stabilization to fragile biomolecules.
Cryo- and Lyoprotectant
BioHale Sucrose, a high purity disaccharide excipient, protects the biologic drugs from the freeze related (cryoprotectant) and drying related (lyoprotectant) stresses. This makes BioHale Sucrose particularly suitable in the stabilization process of today’s biologics.
Product Data
- Description: White, or almost white, crystalline powder, or lustrous, colorless or white, or almost white, crystals
- Source: Plant-derived, isolated from sugar beet
- Molecular Formula: C12H22O11
- Molecular Weight: 342.30 g/mol
- CAS Number: 57-50-1
- Tg: ~61°C
- Manufactured in The Netherlands, Europe
- Multi-compendial specification complies with Ph. Eur., USP-NF, JP, ChP
- Packaging: 20 KG (HDPE drum) with PE inner liner
Product Specifications
Endotoxin |
≤ 0.25 EU/g |
Heavy Metals |
≤ 5 ppm |
Elemental Impurities |
Complies with ICH Q3D |
Total Impurities |
≤ 2.0% |
Reducing sugars |
≤ 0.07% |
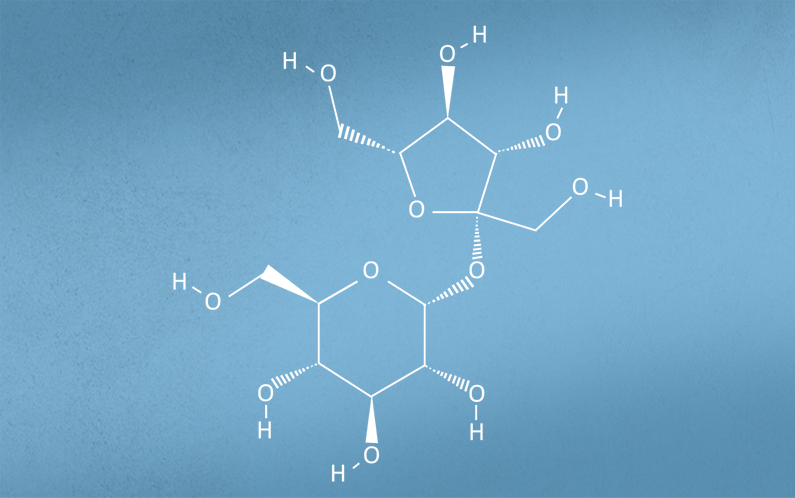

Figure 1. Structural formula of Sucrose
The utility and function are driven by its unique chemical and physical properties especially in aqueous solutions. It provides tonicity, stabilization, cryo-preservation and protection during lyophilization.
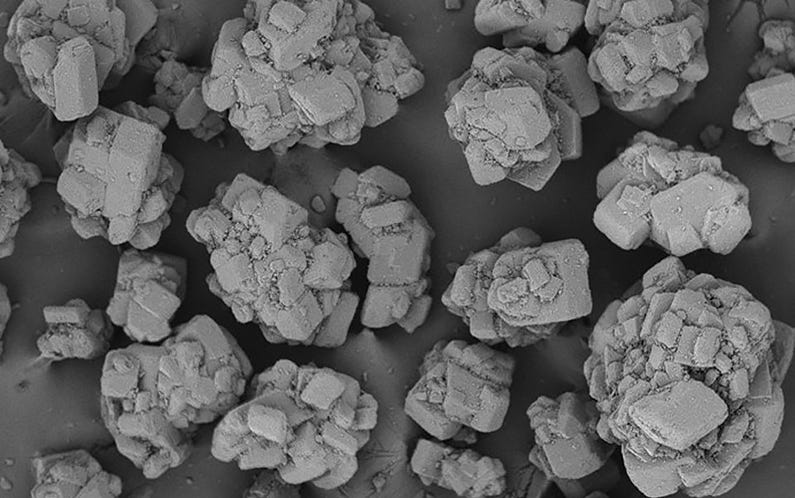

BioHale® Sucrose crystal structure
Product Information
| Chemical Name | Chemical Type | Manufacturer | CAS No. | Catalog No. | Size |
|
BioHale Sucrose (Beet-Derived) USP-NF, EP, JP, ChP Low Endotoxin |
Excipient |
DFE Pharma |
57-50-1 |
1178771 |
20 KG |
BioHale Trehalose Dihydrate
BioHale Trehalose Dihydrate is a disaccharide formed by a 1,1-glycosidic bond between two α-glucose units. It is a non-reducing sugar that is not easily hydrolyzed by acid.
BioHale Trehalose Dihydrate is well suited to provide solution-state stabilization, as well as cryo- and lyo-protection for biomolecules to use in parenteral, inhalation and ophthalmic applications.
Stabilizing Agent
BioHale Trehalose Dihydrate is a non-reducing sugar and as such does not facilitate the Maillard reaction, offering improved compatibility with amino acids and proteins compared with a reducing sugar. BioHale Trehalose provides solution-state stabilization to fragile biomolecules. It is relatively stable under low-pH conditions compared to other disaccharides.
Cryo- and Lyoprotectant
BioHale Trehalose Dihydrate can play an important role in protecting biologics from the freeze and dehydration stresses that occur during lyophilization. Additionally, once in the lyophilized form the high glass transition temperature of BioHale Trehalose Dihydrate can help maintain a glassy matrix and prevent re-crystallization.
Product Data
- Description: White, or almost white, crystals or crystalline powder
- Molecular Formula: C12H22O11 · 2H2O
- Molecular Weight: 378.33 g/mol
- CAS Number: 6138-23-4
- Tg: ~107°C
- Manufactured in The Netherlands, Europe
- Multi-compendial specification complies with Ph. Eur., USP-NF, JP, ChP
- Packaging: 20 KG (HDPE drum) with PE inner liner
Product Specifications
Endotoxin |
≤ 0.30 EU/g |
Heavy Metals |
≤ 5 ppm |
Elemental Impurities |
Complies with ICH Q3D |
Total Impurities |
≤ 0.5% (RRT<1.0) ≤ 0.5% (RRT>1.0) |
Reducing sugars |
≤ 0.1% |
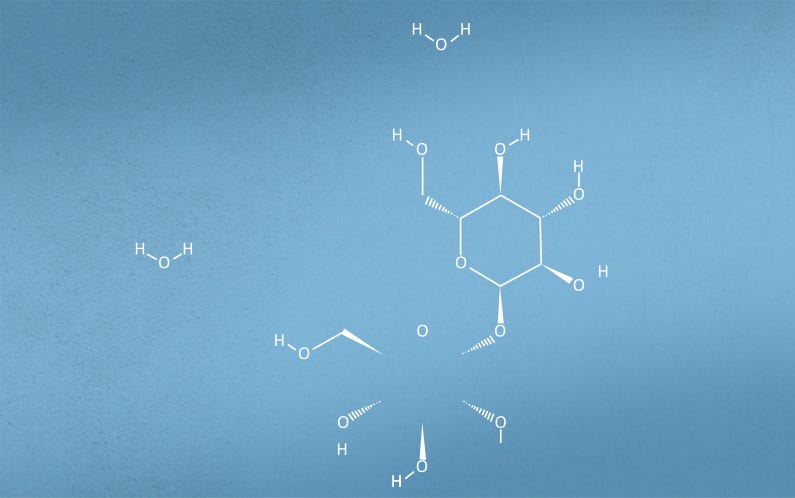

Figure 2. Structural formula of Trehalose Dihydrate
The combination of the molecular structure and physico-chemical properties of Trehalose Dihydrate provides stability to fragile biomolecules in aqueous solutions and during lyophilization.
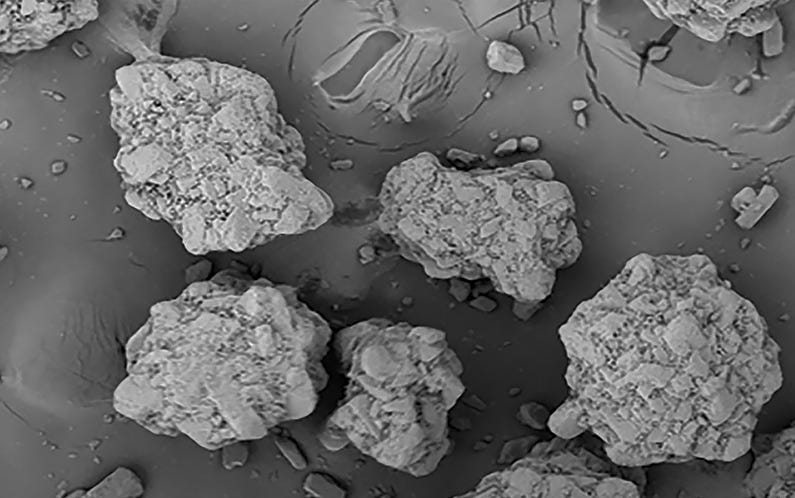

BioHale® Trehalose Dihydrate crystal structure
Product Information
| Chemical Name | Chemical Type | Manufacturer | CAS No. | Catalog No. | Size |
|
BioHale Trehalose Dihydrate USP-NF, EP, JP, ChP Low Endotoxin |
Excipient |
DFE Pharma |
6138-23-4 |
1181599 |
20 KG |





